The Health and Sustainability Science Behind Red-Meat Production and Consumption
Scott Norman
BVSc, PhD, DipACT, GCEd, MANZCVS
This article is provided for information purposes only. There is no explicit, or implicit health advice in this article. Consult a health-care professional for health advice relevant to your personal circumstances.
The goal of this document is to identify peer-reviewed articles to inform a scientific understanding of the role of red meat in human nutrition and health. At the outset, it is suitable to state that the only peer-reviewed, case-control, blinded studies, investigating the long-term influence of dietary red meat (or fat) on human health were – the Polyp Prevention Trial (NIH), (Lanza et al. 2007); the Nurse Health Study (Ardisson Korat, Willett, and Hu 2014); the Minnesota Coronary Experiment (Frantz et al. 1989); and the Sydney Diet Heart Study (Ramsden et al. 2013). It may never be possible to gain further quality data that case-control studies would provide. This is because research welfare and ethics committees would be reluctant to approve such studies, particularly now that World Health Organisation (WHO) doctrine has incriminated red meat as being detrimental to human health in the absence of definitive scientific studies.
Obtaining Reliable Information
Information on the influence of red-meat consumption on human health can arise from:
- Anecdote or opinion
- Non-refereed governmental or industry reports
- Non-refereed reports from industry experts
- Industry funded scientific trials
- Independent, peer-reviewed scientific literature
Peer-reviewed scientific literature published in high-quality journals provides the most reliable information. Peer-review means that at the very least, two or three scientists who were not involved in the data-collection or writing of an article, provide critique of the scientific methods and interpretations of the data. Ideally, studies should be case-controlled. Useful information can come from survey and prospective epidemiological studies, but it needs to be understood that results from epidemiological studies should primarily be used to formulate hypotheses worthy of further investigation utilising higher-level (eg case-control) study designs. For epidemiological studies to be definitive, there must be a very clear demarcation between groups and this is rarely the case.
While it is not the goal of this document to provide in-depth statistical information, it is necessary to have some understanding of the term “Relative Risk” (RR) to comprehend the quality of the epidemiological data upon which some nutritional recommendations have been made. Relative Risk describes the risk of an event (often a disease, injury, risk factor, or death) within one group compared to the risk within another group. The formula for calculating RR is readily available online. But the important point is that if the RR is 1, there is no difference between the two groups. If the RR is greater than 1, there may be an effect causing the difference between the two groups worth investigating with a more definitive trial. The value that can be placed on the RR figure depends on the size of the RR number, the incidence of the condition or event within the populations under investigation, and how data was collected. If the data was collected in well-controlled and managed circumstances (for example an in-patient context where interventions and data collection are performed under the supervision of professionals), a lower RR value will be more relevant compared to data collection that depends on, for example, individuals recalling food intake, or exercise regimens from weeks, months, or years in the past. Additionally, if the incidence of the condition within both populations is low (eg a 2% death-rate in one group, and a 3% death rate in the other group), then RR’s may be misleading. This is because, while there was a 1% increase in death-rate between the groups (which may, or may not, have been due to chance, or problems with inaccurate data collection) there can still be a massive perceived increase in the incidence of the condition between groups. In the example above, a rise in incidence from 2% to 3% translates to a 50% increase in incidence.
An example of where epidemiological data and RR’s have provided definitive evidence is the link between smoking and lung cancer. In this example, the RR of lung-cancer by smoking status was 23.6 (Pesch et al. 2012). Contrast this result with many epidemiological survey-based studies claiming adverse health effects from eating red meat that are based on RR values of between 1.17 and 1.28 (Chan et al. 2011; Larsson and Wolk 2006). There is very little confidence in RR figures less than 2, and when estimates are well below 2, (as is the case with the red meat studies), it is not possible to distinguish between bias, confounding factors and causation (Shapiro 2004). The RR figures of colon cancer by red meat consumption barely justify forming an hypothesis for further study, let alone claiming definitive evidence. Therefore, consideration must always be given to study design, and the type of study that was performed (eg survey/epidemiological data; blinded, controlled study; etc).
Additionally, for all information sources, there needs to be an understanding of whether there were/are any conflicts of interest and whether these were clearly acknowledged within the report or journal article. There also needs to be an understanding of whether there was any implicit, or explicit bias in articles, or reports.
What is Bias?
Bias in relation to research is either an implicit, or explicit favouritism displayed towards one side of a scientific question. An example of an implicit bias is selecting candidates for a trial based on convenience, rather than providing extra effort to fully randomise the grouping of participants. An example of explicit bias is if a group with a financial interest in the outcome of the research funds the trials and insists on being involved in data analysis and reporting. Bias, and conflicts of interest surrounding nutritional research have been described (Mozaffarian 2017), and robustly discussed (Ludwig, Kushi, and Heymsfield 2018). Yet, a point of commonality with all arguments is that there is not enough independent, or government funding for definitive nutritional studies. In an environment with inadequate funding, there is opportunity for industries with vested interests to fund research. This leads to either intended, or unintended bias, and there is a trend to approximately a 30% increase in favourable conclusions for the company providing the funding (Mozaffarian 2017).
Bias can also arise from withholding (not publishing) results from studies when results don’t support a favoured hypothesis. Some would describe bias of this scale as scientific fraud. Particularly if the findings may have widespread health implications. An example of study results being withheld is the Minnesota Coronary Experiment, which ran from 1968 to 1973 (Frantz et al. 1989). This was a well-designed trial, independently funded, with approximately 9,000 participants monitored over 4.5 years. It was designed to determine if diets low in saturated fat and cholesterol reduced the incidence of cardiovascular disease – in essence, comparing diets high in seed oils with diets high in animal-derived fats. The findings from this huge study showed that replacing dietary animal fats with seed oils (mainly linoleic acid) did not reduce the risk of death due to coronary heart disease despite lowering serum cholesterol. Importantly, the study identified a 22% higher risk of death for each 0.78 mmol/L reduction in serum cholesterol. This study was completed in 1973, yet the results were not published until 1989. A second well-designed and run study where results were withheld from publication is the Sydney Diet Heart Study (Woodhill et al. 1978). The study ran from 1966 to 1973, with results published 5 years later. While this is not long compared to the Minnesota Study, the concern is that, even when published, the results were poorly and ambiguously reported. It was not until an independent re-evaluation of the data was performed in 2013, that the results of this large study were made clear (Ramsden et al. 2013). The striking conclusion was, “clinical benefits of the most abundant polyunsaturated fatty acid, omega 6 linoleic acid, have not been established. In this cohort, substituting dietary linoleic acid in place of saturated fats increased the rates of death from all causes, coronary heart disease, and cardiovascular disease. An updated meta-analysis of linoleic acid intervention trials showed no evidence of cardiovascular benefit. These findings could have important implications for worldwide dietary advice to substitute omega 6 linoleic acid, or polyunsaturated fats in general, for saturated fats”.
It would be interesting to know what decisions, documents, reports, or embedded doctrines may have been changed had the information from these two studies been made available in an accurate and timely manner.
An example of an influential source on human nutrition is the World Health Organisation factsheet on Healthy diet (who.int). It is notable that 16 of the 19 “references” for this fact sheet are reports. Implicit in their development, reports will contain the opinions and biases of their authors that are untempered by peer-review. On only 3 occasions is peer-reviewed literature referred to in this WHO factsheet. A scientific critique of the process by which the Healthy diet (who.int) dietary recommendations were reached is provided here – 2020 Dietary Guidelines — The Nutrition Coalition. While there is no assertion that this critique is without fault, it does highlight many concerns regarding the science and interpretations that have led to current nutritional recommendations.
A further concern is that the dietary guidelines of some countries eg Australia and the USA, are based on reports produced by “industry experts” without thorough peer-review, and without a clear statement of potential biases or conflicts of interest. There are several agencies that are influential in the development of nutritional guidelines. Examples include; the International Agency for Research on Cancer (IARC) which is an agency of the World Health Organisation (WHO); the 2020 Dietary Guidelines for America produced by the US government (Home | Dietary Guidelines for Americans); the Harvard T.H. Chan School of Public Health; and the American College of Lifestyle Medicine.
It is beyond the scope of this document to specifically identify contributors to nutritional guidelines and reports who have serious, and often undeclared biases or conflicts. But some straightforward research quickly identifies panellists, authors and researchers who are variably vegan, vegetarian, members of religious groups that recommend a vegetarian lifestyle, or funded in some way by the plant-based food industry. The religious doctrine of one religious group stipulates a plant-based diet, and they founded the American College of Lifestyle Medicine. Their religious beliefs are not under scrutiny here, but the fact this clear bias towards a plant-based diet is not explicitly declared when members contribute to some influential nutritional reports is under scrutiny.
If you are a meat producer, or simply someone who enjoys meat as part of your diet, it is important to have access to high-quality scientific information surrounding your product, or dietary choices. This document aims to provide information directly from peer-reviewed literature on topics of importance surrounding red meat production and consumption.
Conflict of interest statement – Dr Scott Norman is a veterinarian and owner of a grass-fed cattle stud. There has been no industry funding, or incentive to produce the following article or influence its content. To the best of my knowledge and ability, this article represents a critical review of the current scientific knowledge on the topic.
Meat as part of the human diet – The Science
Whenever there is conversation regarding what constitutes a healthy diet, it is common that a robust discussion will ensue. In recent times, the health qualities of dietary red-meat and its production sustainability have been questioned on several levels. Concerns raised regarding red meat consumption include:
- whether meat contains adequate and suitable nutrients for the human diet
- whether the fat content of meat is suitable for healthy human nutrition
- whether the consumption of red meat may contribute to metabolic disease, cancer, or heart disease
- whether there is a difference between grass-fed and grain-fed meat
- whether the production of grass-fed beef contributes significantly to greenhouse gases, or environmental degradation
Disappointingly, most of the scientific studies on nutrition and red meat consumption are based on epidemiological data that has been harvested by surveys. Survey data is inherently unreliable, particularly when questions regarding dietary intake may require participants to provide information from 6 to 12 months in the past. Additionally, many of the identified studies, despite peer-review, have clear errors in data collection and interpretation. An example is an influential paper published from a Nurses Health Study (C. Zhang et al. 2006). The conclusions drawn from this paper made specific reference to the influence of red meat and processed meats on gestational diabetes. Yet, when reading the materials and methods, dietary intake was classified into the following two categories – “The prudent pattern was characterised by a high intake of fruit, green leafy vegetables, poultry and fish, whereas the Western pattern was characterised by high intake of red meat, processed meat, refined grain products, sweets, French fries and pizza.”
There are two serious faults in this statement which would normally result in the article not being accepted for publication. Firstly, the authors have described one of the dietary patterns as “prudent”. This clearly identifies a bias which can either consciously, or subconsciously affect data analysis and interpretation. Secondly, the protein sources of poultry and fish were categorised within the “prudent” diet, alongside fruit and green-leafy vegetables. In contrast, consumption of red meat, or processed meats was categorised with “refined grain products, sweets, French fries, and pizza.” In a study focussed on the investigation of type 2 diabetes mellitus, any researcher with even a basic understanding of the biochemistry and endocrinology of the condition should have understood the irrecoverable conflicts and confounding factors involved. Couple these faults with the vagaries of asking nurses to recall their dietary intakes up to two years previously and asking nurses to self-report whether they had been diagnosed with gestational diabetes, and it becomes apparent that any dietary associations with gestational diabetes become tenuous at best.
Unfortunately, once published, papers such as this can become embedded within the scientific literature. This paper currently has 286 citations.
With these considerations and concerns in mind, the science behind the dot-points above will be explored.
Does meat contain adequate and suitable nutrients for the human diet?
Summary answer – Yes
Red meat contains between 20% to 25% protein, some of which provides amino acids essential for human metabolism. Meat is also a good source of iron, zinc, phosphorous, niacin, riboflavin, vitamin B12, and vitamin B1 (thiamine), (Ahmad, Imran, and Hussain 2018). Red meat also contains a range of fats, the nutritional value of which will be discussed subsequently.
One concern raised regarding a diet high in red meat is the potential to have reduced vitamin C intake. In most animal species, glucose is converted through a series of four reactions to ascorbic acid. In humans, the enzyme for the fourth step, L-gulonolactone oxidase, is inactive. Therefore, the conversion of glucose to ascorbic acid cannot be completed. It has been calculated that if humans had intact glucose/ascorbic acid pathways, they would produce about two to four grams of ascorbic acid per day (Stone 1972). This provides a rough estimate of what the daily human requirement for Vitamin C may be. The reason for providing this information is that it highlights the close relationship between Vitamin C and glucose. Perhaps the most important finding about vitamin C activity is its competition with glucose within the body. In 1975 it was theorised that, because of their structural similarity, vitamin C and glucose might utilise the same membrane transport (Mann and Newton 1975). This theory was eventually confirmed experimentally (Bigley et al. 1983; Padh, Subramoniam, and Aleo 1985; Som et al. 1981; Chen et al. 1983) and ultimately led to an understanding of how glucose and vitamin C compete for transport by insulin and entry into cells (Cunningham 1988). The upshot of this is that in diets high in glucose, there seems to be a need for higher vitamin C intake. In contrast, diets low in sugars result in a reduced need for vitamin C. Therefore, diets high in meat and low in sugar will result in a reduced need for vitamin C, with a resultant reduced chance of deficiency.
Is the fat content of meat suitable for healthy human nutrition?
Summary answer – Yes.
The fatty acid composition of meat will vary by animal age, sex, breed, diet and within the cut of meat (Wood and Enser 1997). Despite general doctrine that red meat contains mainly saturated fat, the literature identifies the breakdown of fats in steak from cattle to be approximately one third oleic acid (like olive oil), one third polyunsaturated fats, and one third saturated fats (McAfee et al. 2010).
Of this fat profile, saturated fats have been the subject of a lot of negative reports regarding their effect on human health. The hypothesis that saturated fats and dietary cholesterol cause heart attacks and strokes – the so-called diet heart hypothesis proposed decades ago by Ancel Keys – was one of the main reasons red meat was rejected as a healthy food source (Page et al. 1961). However, this hypothesis has now been tested on around 75,655 men and women in trials lasting from one to 12 years. Most of these trials involved in-patient candidates, and so diets and cases were well controlled. Most of the trials were also Government funded, thus removing possible bias as may occur with industry funding. Results over all these studies found no adverse effect of saturated fats on cardiovascular mortality, or total mortality (Nina Teicholz). These results should have put an end to any concerns regarding red meat consumption, but unfortunately they have been buried under the weight of pre-existing doctrine built up over the previous 60 years.
Can red-meat consumption contribute to metabolic disease, cancer, or heart disease?
Metabolic disease
Diabetes is the main metabolic disease where there is doctrine that disease incidence may be reduced by plant-based diets (WHO). The clear point to make on these reports is that there are no definitive blinded-control, prospective studies within the peer-reviewed, scientific literature that specifically investigate the influence of red-meat consumption on metabolic disease. All the negative comments regarding red meat consumption on metabolic disease are based on survey data.
Yet, purines contained in meat can be converted to uric acid following digestion. There is some evidence that uric acid may be involved in fructose-induced metabolic syndrome (R. J. Johnson et al. 2009). The important understanding is that for uric acid to influence the progress of metabolic syndrome and diabetes, the metabolic syndrome must first be induced by excessive fructose intake. The inference is that the consumption of meat in the absence of a high-fructose diet will not contribute to the development of metabolic syndrome.
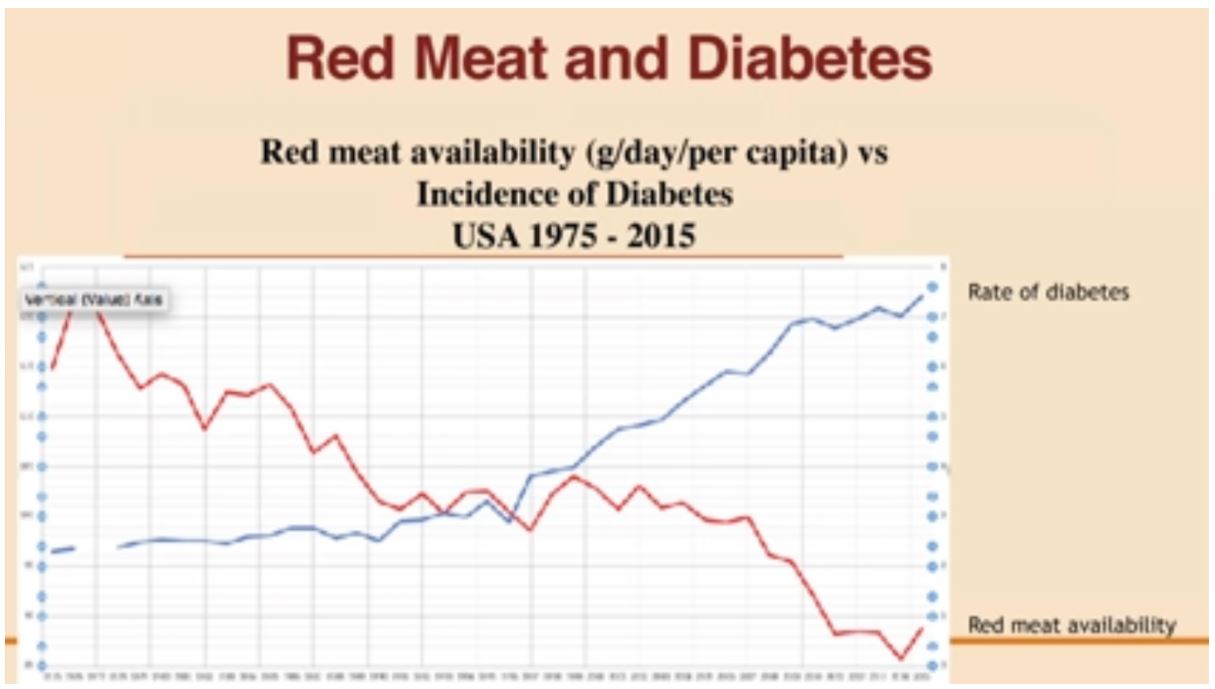
The chart summarised below provides powerful evidence of a negative correlation between red meat consumption and diabetes. It is based on 40 years of data and clearly shows the trend of increasing diabetes incidence as the availability and consumption of red-meat decreases (Teicholz 2019).
Red meat and cancer – what is the evidence?
The only case-control study identified that explored this topic is the Polyp Prevention Trial run by the American National Institute of Health (NIH) (Lanza et al. 2007). The NIH is an independent body that should be exposed to little bias. This study initially ran for four years with 1,905 participants. There was a further follow-up another 4 years later with 801 of the original participants still available for this 8-year assessment. While not specifically focused on red meat intake, the trial investigated whether a high-fibre (18 g/1,000 kcal), high-fruit and-vegetable (3.5 servings/1,000 kcal), and low-fat (20% of total energy) diet would reduce the recurrence of adenomatous polyps in the large bowel. This study failed to show any effect of a low-fat, high-fibre, high-fruit and-vegetable eating pattern on adenoma recurrence even with 8 years of follow-up (Lanza et al. 2007).
In contrast to the single case-control study mentioned above, there are many epidemiological survey-based studies claiming that eating red meat may cause cancer (Larsson and Wolk 2006; Chan et al. 2011). These conclusions are based on RR values between 1.17 and 1.28 and as noted previously, when RR estimates are well below 2, it is not possible to distinguish between bias, confounding factors and causation (Shapiro 2004). These RR figures of colon cancer by red meat consumption barely justify forming an hypothesis for further study, let alone claiming definitive evidence.
Coupling the findings of the Polyp Prevention Trial with those of the epidemiological studies provides no evidence that red meat causes colon cancer. In further support of this conclusion, a 2017 literature review was performed following the IARC claim that red meat was carcinogenic (I. T. Johnson 2017). Following this extensive review, it was concluded that: “epidemiology indicates that processed meat products are associated with increased risk of colorectal cancer. Yet, evidence for red meat and for other cancers remains tentative” (I. T. Johnson 2017). This conclusion is carefully worded but does not disguise the fact that there was no definitive evidence identified for red meat being a cause of cancer.
Red meat and heart disease – what is the evidence?
As noted above, red meat has a range of fats. Depending on animal management, the literature suggests the fat profile to be approximately one third oleic acid (like olive oil), one third polyunsaturated fats, and one third saturated fats (McAfee et al. 2010). Yet doctrine focuses on meat containing mainly saturated fats. Despite this, the hypothesis that saturated fats and dietary cholesterol cause heart attacks and strokes – the so-called “diet heart hypothesis” proposed decades ago by Ancel Keys – was one of the main reasons red meat was rejected as a healthy food source (Page et al. 1961). However, the “diet heart hypothesis” has now been tested on around 75,655 men and women in trials lasting from one to 12 years. Most of these trials involved in-patient candidates, and so diets and cases were well controlled (Woodhill et al. 1978; Frantz et al. 1989; Lanza et al. 2007; Larsson and Wolk 2006; McAfee et al. 2010; I. T. Johnson 2017; Ramsden et al. 2013). Most of the trials were also Government funded, thus removing possible bias as may occur with industry funding. Results over all these studies found no adverse effect of saturated fats on cardiovascular mortality, or total mortality (Teicholz N 2019). These results should have put an end to any concerns regarding red meat consumption, and cardiovascular disease.
Grass-Fed meat or Grain-Fed meat – what is the difference?
For good flavour, the marbling fat content of meat should be above 30% (Wood et al. 1999). The composition of these fats is influenced by the diet. There is a more favourable omega-6 to omega-3 fatty acid ratio, for grass-fed cattle (2.0–2.5:1.0), compared to grain-fed cattle (8–30:1). Therefore, grass-fed beef contains significantly more omega-3 fatty acids compared to grain-fed beef (Hall, Schönfeldt, and Pretorius 2016; Wood et al. 1999; Schönfeldt and Hall 2015). While a review of the benefits of higher omega-3 fatty acids in the diet is the topic for another review, the summary is that high dietary omega-3 intake leads to less inflammatory conditions compared to diets higher in omega-6’s. This comment is also relevant when considering the high omega-6 content of dietary seed-oils (incorrectly referred to as “vegetable” oils) which are commonly used for cooking and frying.
Greenhouse gas production and environmental degradation – a comparison between grass-fed beef production and broadacre cropping
This Section is a work in progress. While there are studies and views on the environmental impact of beef production, there seems to be little information with transparent calculations on the environmental impact of broadacre grain production in Australia. Additionally, there is a need to understand the effect of wastage from the different food sectors on their environmental impact. Preliminary reading suggests there is an overall wastage (including pre and post sale) of approximately 30%. Initial impressions from the literature suggest there is a higher proportion of pre-sale waste from the plant-based foods due to cosmetic and taste spoilage. In the post-sale period, it seems consumers are less-likely to waste animal-based food compared to plant-based foods. There is a need for further review in this space. In the meantime, some useful references have been identified. Note that some of these references persist with the view that red meat consumption is detrimental to health. This view-point is contested.
(Röös, Sundberg, and Hansson 2011)
(Peters et al. 2010)
(van der Weele et al. 2019)
What are the benefits in eating red meat?
Red meat is a low-joule (calorie) source of high-quality protein. It contains between 20% to 25% protein, and is one of only a few sources providing some amino acids which are essential for human metabolism. Meat is also a good source of iron, zinc, niacin, riboflavin, vitamin B12, vitamin B1 (thiamine) and selenium. This contrasts with legume-based foods that are known to bind iron, zinc and calcium, reducing their absorption into the body (Y. Y. Zhang et al. 2020)
Approximately 66% of the fats in red meat are either polyunsaturated, or monounsaturated fats, with grass-fed beef providing a higher portion of healthy omega-3 fatty acids. While a third of the fats are saturated, there is still conjecture within the literature as to the effect of saturated animal-fat intake on cardiovascular disease, cancer and metabolic disease. Importantly, there is evidence that saturated fats may provide human health benefits, in contrast to the high linoleic acid content of many seed oils.
In Summary
There is no strong evidence that red meat consumption causes ill-health. There is also no strong evidence that avoiding red meat will improve health. To the contrary, there is firm evidence that replacing animal fats with seed oils (mainly linoleic acid) is detrimental to health. The deceptive marketing employed by manufacturers of highly processed seed oils (marketed as “vegetable” oils) is concerning. This marketing is aimed at conveying a healthy image for seed-oils despite strong evidence they are detrimental to human health. Unfortunately, after exploring the literature, there is evidence that a large body of work leading to current nutritional recommendations surrounding red meat consumption has been based on either biased data, biased data interpretation, or poorly informed data interpretation. It is also concerning that the results of two seminal studies (the Minnesota and Sydney studies) were either withheld, or ambiguously reported prior to the 1980 release of the first Dietary Guidelines in the USA. These dietary guidelines and their subsequent iterations provide the basis for all US nutrition policies, and food assistance programs, including educational programs, Department of Defense, Department of Corrections, aged care, hospital care and medical recommendations. It is not possible for a worker within any of these institutions to deviate from the government recommendations regardless of whether there may be scientific evidence to the contrary. Unfortunately, these US recommendations appear to have influenced nutritional guidelines throughout the world.
Finally, there is evidence that red meat consumption supplies some essential nutrients to the human diet that are difficult to obtain from other sources.
Bibliography
Ahmad, Rabia Shabir, Ali Imran, and Muhammad Bilal Hussain. 2018. “Nutritional Composition of Meat.” In Meat Science and Nutrition. https://doi.org/10.5772/intechopen.77045.
Ardisson Korat, Andres v., Walter C. Willett, and Frank B. Hu. 2014. “Diet, Lifestyle, and Genetic Risk Factors for Type 2 Diabetes: A Review from the Nurses’ Health Study, Nurses’ Health Study 2, and Health Professionals’ Follow-Up Study.” Current Nutrition Reports. https://doi.org/10.1007/s13668-014-0103-5.
Bigley, R., M. Wirth, D. Layman, M. Riddle, and L. Stankova. 1983. “Interaction between Glucose and Dehydroascorbate Transport in Human Neutrophils and Fibroblasts.” Diabetes 32 (6 I). https://doi.org/10.2337/diab.32.6.545.
Chan, Doris S.M., Rosa Lau, Dagfinn Aune, Rui Vieira, Darren C. Greenwood, Ellen Kampman, and Teresa Norat. 2011. “Red and Processed Meat and Colorectal Cancer Incidence: Meta-Analysis of Prospective Studies.” PLoS ONE 6 (6). https://doi.org/10.1371/journal.pone.0020456.
Chen, M. S., M. L. Hutchinson, R. E. Pecoraro, W. Y. Lee, and R. F. Labbe. 1983. “Hyperglycemia-Induced Intracellular Depletion of Ascorbic Acid in Human Mononuclear Leukocytes.” Diabetes 32 (11). https://doi.org/10.2337/diabetes.32.11.1078.
Cunningham, John J. 1988. “Altered Vitamin C Transport in Diabetes Mellitus.” Medical Hypotheses 26 (4). https://doi.org/10.1016/0306-9877(88)90131-4.
Frantz, I. D., E. A. Dawson, P. L. Ashman, L. C. Gatewood, G. E. Bartsch, K. Kuba, and E. R. Brewer. 1989. “Test of Effect of Lipid Lowering by Diet on Cardiovascular Risk. The Minnesota Coronary Survey.” Arteriosclerosis 9 (1). https://doi.org/10.1161/01.atv.9.1.129.
Hall, Nicolestte, Hettie Carina Schönfeldt, and Beulah Pretorius. 2016. “Fatty Acids in Beef from Grain- and Grass-Fed Cattle: The Unique South African Scenario.” South African Journal of Clinical Nutrition 29 (2). https://doi.org/10.1080/16070658.2016.1216359.
Johnson, Ian T. 2017. “The Cancer Risk Related to Meat and Meat Products.” British Medical Bulletin 121 (1). https://doi.org/10.1093/bmb/ldw051.
Johnson, Richard J., Santos E. Perez-Pozo, Yuri Y. Sautin, Jacek Manitius, Laura Gabriela Sanchez-Lozada, Daniel I. Feig, Mohamed Shafiu, et al. 2009. “Hypothesis: Could Excessive Fructose Intake and Uric Acid Cause Type 2 Diabetes?” Endocrine Reviews 30 (1). https://doi.org/10.1210/er.2008-0033.
Lanza, Elaine, Binbing Yu, Gwen Murphy, Paul S. Albert, Bette Caan, James R. Marshall, Peter Lance, et al. 2007. “The Polyp Prevention Trial-Continued Follow-up Study: No Effect of a Low-Fat, High-Fiber, High-Fruit, and -Vegetable Diet on Adenoma Recurrence Eight Years after Randomization.” Cancer Epidemiology Biomarkers and Prevention 16 (9). https://doi.org/10.1158/1055-9965.EPI-07-0127.
Larsson, Susanna C., and Alicja Wolk. 2006. “Meat Consumption and Risk of Colorectal Cancer: A Meta-Analysis of Prospective Studies.” International Journal of Cancer 119 (11). https://doi.org/10.1002/ijc.22170.
Ludwig, David S., Lawrence H. Kushi, and Steven B. Heymsfield. 2018. “Conflicts of Interest in Nutrition Research[1].” JAMA – Journal of the American Medical Association. https://doi.org/10.1001/jama.2018.5658.
Mann, George v., and Pamela Newton. 1975. “THE MEMBRANE TRANSPORT OF ASCORBIC ACID.” Annals of the New York Academy of Sciences 258 (1). https://doi.org/10.1111/j.1749-6632.1975.tb29285.x.
McAfee, Alison J., Emeir M. McSorley, Geraldine J. Cuskelly, Bruce W. Moss, Julie M.W. Wallace, Maxine P. Bonham, and Anna M. Fearon. 2010. “Red Meat Consumption: An Overview of the Risks and Benefits.” Meat Science 84 (1). https://doi.org/10.1016/j.meatsci.2009.08.029.
Mozaffarian, Dariush. 2017. “Conflict of Interest and the Role of the Food Industry in Nutrition Research.” JAMA – Journal of the American Medical Association. https://doi.org/10.1001/jama.2017.3456.
Padh, Harish, A. Subramoniam, and Joseph J. Aleo. 1985. “Glucose Inhibits Cellular Ascorbic Acid Uptake by Fibroblasts in Vitro.” Cell Biology International Reports 9 (6). https://doi.org/10.1016/0309-1651(85)90017-7.
Page, Irvine H., Edgar v. Allen, Francis L. Chamberlain, Ancel Keys, Jeremiah Stamler, and Fredrick J. Stare. 1961. “Dietary Fat and Its Relation to Heart Attacks and Strokes.” Circulation 23 (1). https://doi.org/10.1161/01.cir.23.1.133.
Pesch, Beate, Benjamin Kendzia, Per Gustavsson, Karl Heinz Jöckel, Georg Johnen, Hermann Pohlabeln, Ann Olsson, et al. 2012. “Cigarette Smoking and Lung Cancer – Relative Risk Estimates for the Major Histological Types from a Pooled Analysis of Case – Control Studies.” International Journal of Cancer 131 (5). https://doi.org/10.1002/ijc.27339.
Peters, Gregory M., Hazel v. Rowley, Stephen Wiedemann, Robyn Tucker, Michael D. Short, and Matthias Schulz. 2010. “Red Meat Production in Australia: Life Cycle Assessment and Comparison with Overseas Studies.” Environmental Science and Technology 44 (4). https://doi.org/10.1021/es901131e.
Ramsden, Christopher E., Daisy Zamora, Boonseng Leelarthaepin, Sharon F. Majchrzak-Hong, Keturah R. Faurot, Chirayath M. Suchindran, Amit Ringel, John M. Davis, and Joseph R. Hibbeln. 2013. “Use of Dietary Linoleic Acid for Secondary Prevention of Coronary Heart Disease and Death: Evaluation of Recovered Data from the Sydney Diet Heart Study and Updated Meta-Analysis.” BMJ (Online) 346 (7894). https://doi.org/10.1136/bmj.e8707.
Röös, Elin, Cecilia Sundberg, and Per Anders Hansson. 2011. “Uncertainties in the Carbon Footprint of Refined Wheat Products: A Case Study on Swedish Pasta.” International Journal of Life Cycle Assessment 16 (4). https://doi.org/10.1007/s11367-011-0270-1.
Schönfeldt, H. C., and N. Hall. 2015. “Nutrient Content of South African Red Meat and the Effect of Age and Production System.” South African Journal of Animal Sciences 45 (3). https://doi.org/10.4314/sajas.v45i3.9.
Shapiro, Samuel. 2004. “Looking to the 21st Century: Have We Learned from Our Mistakes, or Are We Doomed to Compound Them?” Pharmacoepidemiology and Drug Safety 13 (4). https://doi.org/10.1002/pds.903.
Som, S., S. Basu, D. Mukherjee, S. Deb, P. Roy Choudhury, S. Mukherjee, S. N. Chatterjee, and I. B. Chatterjee. 1981. “Ascorbic Acid Metabolism in Diabetes Mellitus.” Metabolism 30 (6). https://doi.org/10.1016/0026-0495(81)90133-5.
Stone, I. 1972. “The Natural History of Ascorbic Acid in the Evolution of the Mammals and Primates and Its Significance for Present Day Man.” ORTHOMOLEC.PSYCHIAT. 1 (2–3).
Weele, Cor van der, Peter Feindt, Atze Jan van der Goot, Barbara van Mierlo, and Martinus van Boekel. 2019. “Meat Alternatives: An Integrative Comparison.” Trends in Food Science and Technology 88. https://doi.org/10.1016/j.tifs.2019.04.018.
Wood, J. D., and M. Enser. 1997. “Factors Influencing Fatty Acids in Meat and the Role of Antioxidants in Improving Meat Quality.” British Journal of Nutrition 78 (1). https://doi.org/10.1079/bjn19970134.
Wood, J. D., M. Enser, A. v. Fisher, G. R. Nute, R. I. Richardson, and P. R. Sheard. 1999. “Manipulating Meat Quality and Composition.” Proceedings of the Nutrition Society 58 (2). https://doi.org/10.1017/s0029665199000488.
Woodhill, J. M., A. J. Palmer, B. Leelarthaepin, C. McGilchrist, and R. B. Blacket. 1978. “Low Fat, Low Cholesterol Diet in Secondary Prevention of Coronary Heart Disease.” Advances in Experimental Medicine and Biology 109. https://doi.org/10.1007/978-1-4684-0967-3_18.
Zhang, C, M B Schulze, C G Solomon, and F B Hu. 2006. “A Prospective Study of Dietary Patterns, Meat Intake and the Risk of Gestational Diabetes Mellitus.” Diabetologia 49 (11): 2604–13. https://doi.org/10.1007/s00125-006-0422-1.
Zhang, Yianna Y., Regine Stockmann, Ken Ng, and Said Ajlouni. 2020. “Revisiting Phytate-Element Interactions: Implications for Iron, Zinc and Calcium Bioavailability, with Emphasis on Legumes.” Critical Reviews in Food Science and Nutrition. https://doi.org/10.1080/10408398.2020.1846014.